Nir Waiskopf, Ben-Shahar, Yuval , Galchenko, Michael , Carmel, Inbal , Moshitzky, Gilli , Soreq, Hermona , and Banin, Uri . 2016.
“Photocatalytic Reactive Oxygen Species Formation By Semiconductor&Ndash;Metal Hybrid Nanoparticles. Toward Light-Induced Modulation Of Biological Processes”. Nano Letters, 16, 7, Pp. 4266-4273. .
Publisher's Version Abstract  Semiconductor–metal hybrid nanoparticles manifest efficient light-induced spatial charge separation at the semiconductor–metal interface, as demonstrated by their use for hydrogen generation via water splitting. Here, we pioneer a study of their functionality as efficient photocatalysts for the formation of reactive oxygen species. We observed enhanced photocatalytic activity forming hydrogen peroxide, superoxide, and hydroxyl radicals upon light excitation, which was significantly larger than that of the semiconductor nanocrystals, attributed to the charge separation and the catalytic function of the metal tip. We used this photocatalytic functionality for modulating the enzymatic activity of horseradish peroxidase as a model system, demonstrating the potential use of hybrid nanoparticles as active agents for controlling biological processes through illumination. The capability to produce reactive oxygen species by illumination on-demand enhances the available peroxidase-based tools for research and opens the path for studying biological processes at high spatiotemporal resolution, laying the foundation for developing novel therapeutic approaches.
Semiconductor–metal hybrid nanoparticles manifest efficient light-induced spatial charge separation at the semiconductor–metal interface, as demonstrated by their use for hydrogen generation via water splitting. Here, we pioneer a study of their functionality as efficient photocatalysts for the formation of reactive oxygen species. We observed enhanced photocatalytic activity forming hydrogen peroxide, superoxide, and hydroxyl radicals upon light excitation, which was significantly larger than that of the semiconductor nanocrystals, attributed to the charge separation and the catalytic function of the metal tip. We used this photocatalytic functionality for modulating the enzymatic activity of horseradish peroxidase as a model system, demonstrating the potential use of hybrid nanoparticles as active agents for controlling biological processes through illumination. The capability to produce reactive oxygen species by illumination on-demand enhances the available peroxidase-based tools for research and opens the path for studying biological processes at high spatiotemporal resolution, laying the foundation for developing novel therapeutic approaches.
Jing Liu, Amit, Yorai , Li, Yuanyuan , Plonka, Anna M, Ghose, Sanjit , Zhang, Lihua , Stach, Eric A, Banin, Uri , and Frenkel, Anatoly I. 2016.
“Reversed Nanoscale Kirkendall Effect In Au&Ndash;Inas Hybrid Nanoparticles”. Chemistry Of Materials, 28, 21, Pp. 8032-8043. .
Publisher's Version Abstract 
Metal–semiconductor hybrid nanoparticles (NPs) offer interesting synergistic properties, leading to unique behaviors that have already been exploited in photocatalysis, electrical, and optoelectronic applications. A fundamental aspect in the synthesis of metal–semiconductor hybrid NPs is the possible diffusion of the metal species through the semiconductor lattice. The importance of understanding and controlling the co-diffusion of different constituents is demonstrated in the synthesis of various hollow-structured NPs via the Kirkendall effect. Here, we used a postsynthesis room-temperature reaction between AuCl3 and InAs nanocrystals (NCs) to form metal–semiconductor core–shell hybrid NPs through the “reversed Kirkendall effect”. In the presented system, the diffusion rate of the inward diffusing species (Au) is faster than that of the outward diffusing species (InAs), which results in the formation of a crystalline metallic Au core surrounded by an amorphous, oxidized InAs shell containing nanoscale voids. We used time-resolved X-ray absorption fine-structure (XAFS) spectroscopy to monitor the diffusion process and found that both the size of the Au core and the extent of the disorder of the InAs shell depend strongly on the Au-to-NC ratio. We have determined, based on multielement fit analysis, that Au diffuses into the NC via the kick-out mechanism, substituting for In host atoms; this compromises the structural stability of the lattice and triggers the formation of In–O bonds. These bonds were used as markers to follow the diffusion process and indicate the extent of degradation of the NC lattice. Time-resolved X-ray diffraction (XRD) was used to measure the changes in the crystal structures of InAs and the nanoscale Au phases. By combining the results of XAFS, XRD, and electron microscopy, we correlated the changes in the local structure around Au, As, and In atoms and the changes in the overall InAs crystal structure. This correlative analysis revealed a co-dependence of different structural consequences when introducing Au into the InAs NCs. Therefore, this study of diffusion effects in nanocrystals has relevance to powerful concepts in solid-state nanochemistry related to processes of cation exchange, doping reactions, and diffusion mechanisms.

The level structure of copper sulfide nanocrystals of different sizes was investigated by correlating scanning tunneling spectroscopy and cyclic voltammetry data in relation to sensing applications. Upon oxidation of Cu2S nanocrystals in the low‐chalcocite phase, correlated changes are detected by both methods. The cyclic voltammetry oxidation peak of Cu(1+) down shifts, while in‐gap states, adjacent to the valence‐band edge, appeared in the tunneling spectra. These changes are attributed to Cu vacancy formation leading to a Cu depleted phase of the nanocrystals. The relevance of the oxidation to the use of copper sulfide nanocrystals in hydrogen peroxide sensing was also addressed, showing that upon oxidation the sensitivity vanishes. These findings bare significance to the use of copper sulfide nanocrystals in glucose sensing applications.
Yuval Ben-Shahar, Scotognella, Francesco , Kriegel, Ilka , Moretti, Luca , Cerullo, Giulio , Rabani, Eran , and Banin, Uri . 2016.
“Optimal Metal Domain Size For Photocatalysis With Hybrid Semiconductor-Metal Nanorods”. Nature Communications, 7, Pp. 10413. .
Publisher's Version Abstract 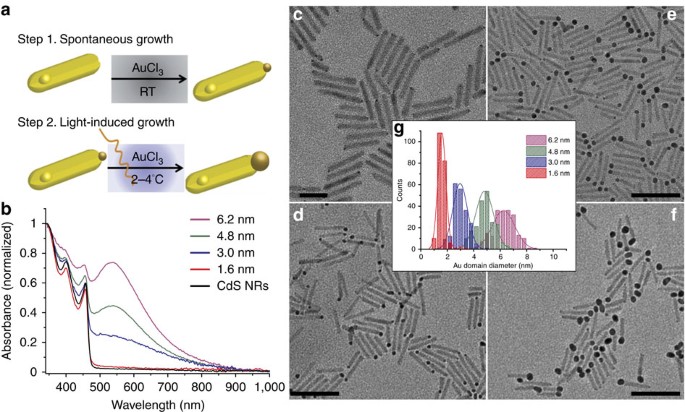
Semiconductor-metal hybrid nanostructures offer a highly controllable platform for light-induced charge separation, with direct relevance for their implementation in photocatalysis. Advances in the synthesis allow for control over the size, shape and morphology, providing tunability of the optical and electronic properties. A critical determining factor of the photocatalytic cycle is the metal domain characteristics and in particular its size, a subject that lacks deep understanding. Here, using a well-defined model system of cadmium sulfide-gold nanorods, we address the effect of the gold tip size on the photocatalytic function, including the charge transfer dynamics and hydrogen production efficiency. A combination of transient absorption, hydrogen evolution kinetics and theoretical modelling reveal a non-monotonic behaviour with size of the gold tip, leading to an optimal metal domain size for the most efficient photocatalysis. We show that this results from the size-dependent interplay of the metal domain charging, the relative band-alignments, and the resulting kinetics.
